Risk profile Ar, Ch, En, Fr, Ru, Sp (PDF)
Risk management evaluation Ar, Ch, En, Fr, Ru, Sp (PDF)
Chemical identity and properties
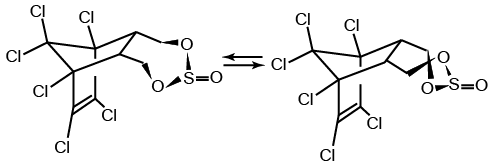
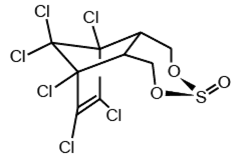
Endosulfan occurs as two isomers: alpha- and beta-endosulfan. They are both biologically active. Technical endosulfan (CAS No: 115-29-7) is a mixture of the two isomers along with small amounts of impurities.
|
|
alpha-endosulfan
CAS No: 959-98-8
|
|
beta-endosulfan
CAS No: 33213-65-9
|
Use and production
According to the risk management evaluation on endosulfan, adopted by the POPRC, endosulfan is an insecticide that has been used since the 1950s to control crop pests, tsetse flies and ectoparasites of cattle and as a wood preservative. As a broad-spectrum insecticide, endosulfan is currently used to control a wide range of pests on a variety of crops including coffee, cotton, rice, sorghum and soy.
A total of between 18,000 and 20,000 tons of endosulfan are produced annually in Brazil, China, India, Israel and South Korea. Colombia, the United States of America and several countries in Europe that used to produce endosulfan have stopped its production.
The largest users of endosulfan (Argentina, Australia, Brazil, China, India, Mexico, Pakistan and the United States) use a total of about 15,000 tons of endosulfan annually. An additional 21 countries report using endosulfan. The use of endosulfan is banned or will be phased out in 60 countries that, together, account for 45 per cent of current global use.
POPs characteristics of endosulfan
According to the risk profile on endosulfan, adopted by the POPRC, endosulfan is persistent in the atmosphere, sediments and water. Endosulfan bioaccumulates and has the potential for long-range transport. It has been detected in air, sediments, water and in living organisms in remote areas, such as the Arctic, that are distant from areas of intensive use.
Endosulfan is toxic to humans and has been shown to have adverse effects on a wide range of aquatic and terrestrial organisms. Exposure to endosulfan has been linked to congenital physical disorders, mental retardations and deaths in farm workers and villagers in developing countries in Africa, Asia and Latin America. Endosulfan sulfate shows toxicity similar to that of endosulfan.
Replacement of endosulfan
Chemical and non-chemical alternatives to endosulfan are available in many geographical situations both in developed and developing countries. Some of these alternatives are being applied in countries where endosulfan has been banned or is being phased-out. However, in some countries, it may be difficult and/or costly to replace endosulfan for specific crop-pest complexes. Some countries also prefer to use endosulfan in pollinator management, insecticide resistance management, integrated pest management systems and because it is effective against a broad range of pests. Some countries want to continue to use endosulfan to allow time for the phase-in of alternatives.
For more information, please refer to the technical endosulfan page.