Pentachlorophenol (PCP) and its salts and esters are lited in Annex A with specific exemptions for use in utility poles and cross-arms (deicison SC-7/3).
|
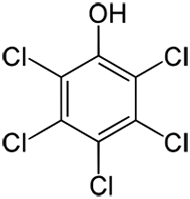
CAS No:
No: 87-86-5 (Pentachlorophenol)
No: 131-52-2 (sodium pentachlorophenate)
No: 27735-64-4 (as monohydrate)
No: 3772-94-9 (pentachlorophenyl laurate)
No: 1825-21-4 (pentachloroanisole) |
Use
PCP has been used as herbicide, insecticide, fungicide, algaecide, disinfectant and as an ingredient in antifouling paint. Some applications were in agricultural seeds, leather, wood preservation, cooling tower water, rope and paper mill system. Its use has been significantly declined due to the high toxicity of PCP and its slow biodegradation.
Production
First produced in the 1930s, it is marketed under many trade names. The main contaminants include other polychlorinated phenols, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzo furans.
Toxicity
People may be exposed to PCP in occupational settings through the inhalation of contaminated workplace air and dermal contact or with wood products treated with PCP. Short-term exposure to large amounts of PCP can cause harmful effects on the liver, kidneys, blood, lungs, nervous system, immune system, and gastrointestinal tract. Elevated temperature, profuse sweating, uncoordinated movement, muscle twitching, and coma are additional side effects. Contact with PCP can irritate the skin, eyes, and mouth. Long-term exposure to low levels such as those that occur in the workplace can cause damage to the liver, kidneys, blood, and nervous system. Finally exposure to PCP is also associated with carcinogenic, renal, and neurological effects.
Alternatives
Both chemical and non-chemical alternatives exist for PCP within applications for utility poles and cross arms. For me information, please refer to the alternatives to PCP page.